FDA 2025 Front-of-Package Labeling Rule: What Beverage Exporters Must Know

Overview of the FDA’s Front-of-Package Labeling Rule in 2025
On January 16, 2025, the U.S. Food and Drug Administration (FDA) proposed a Front-of-Package Labeling Rule (FOP) requiring key nutrition facts such as calories, added sugars, sodium, and saturated fat to appear on the front panel of packaged food and beverage products.
This is designed to improve public health outcomes by making nutritional information more accessible and transparent at the point of purchase.
What Must Be Displayed on the Front of the Package?
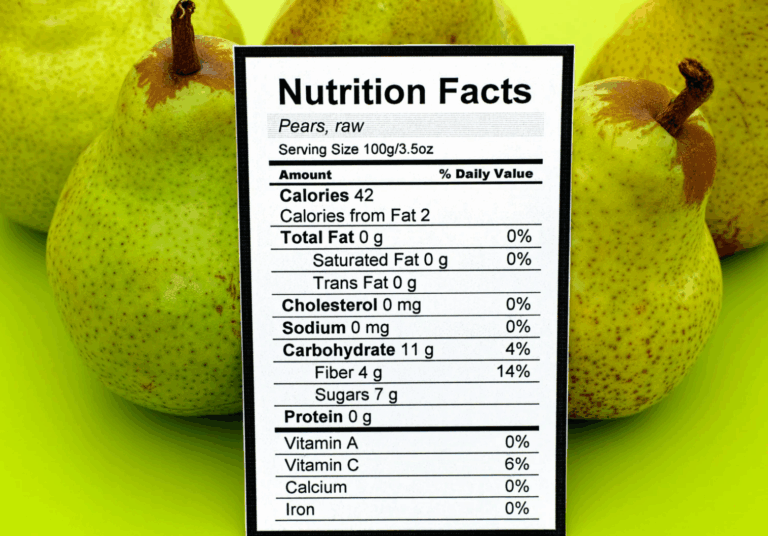
Under the proposed rule, manufacturers and exporters must clearly label the following per serving:
- Calories
- Total and Added Sugars
- Sodium
- Saturated Fat
The label must be easy to read, placed on the principal display panel, and comply with size, contrast, and placement requirements specified by the FDA.
Who Is Affected by the Rule?
The rule applies to:
- All domestic and imported packaged food and beverage products sold in the U.S.
- Particularly relevant to juice, functional beverages, and cereal drinks, which often carry health-related marketing claims.
- OEM and ODM brands manufacturing for private labels will also be required to comply before distribution.
Impacts on Beverage Exporters
This rule could significantly impact exporters in the following ways:
- Reformulation: Brands may need to reduce sugar or sodium to avoid negative impressions.
- Relabeling Costs: All U.S.-bound SKUs must update packaging.
- Customs Clearance Delays: Products not properly labeled may face delays or refusal.
- Market Access Risks: Non-compliance could result in delisting or regulatory holds by the FDA.
What Exporters Should Do Now
✅ Nutritional Assessment: Analyze current formulations and nutrient declarations.
✅ Packaging Audit: Redesign front labels to comply with FOP layout, size, and contrast requirements.
✅ Documentation Sync: Ensure shipping, FDA Prior Notice, and e-commerce listings match the FOP info.
✅ Consult Experts: Especially for OEMs/ODMs unfamiliar with U.S. regulatory nuances.
The FDA’s proposed 2025 front-of-package labeling rule marks a major shift in how nutrition transparency is regulated for food and beverage products sold in the U.S. For juice and functional beverage exporters, this means more than just a label change—it’s a compliance milestone that affects product development, documentation, and market access. Now is the time to evaluate your formulations, update packaging, and align with FOP labeling requirements to avoid costly delays or regulatory pushback when entering the U.S. market.
Stay ahead with curated insights from verified global sources. Follow Nam Viet Group for timely, trustworthy updates on international F&B export regulations, certification trends, and labeling standards.
Source: federalregister.gov